Abstract
Background: Patients (pts) with secondary acute myeloid leukemia (s-AML) have poor long-term outcomes following standard induction chemotherapy with 7+3. Last year, a liposomal cytarabine and daunorubicin formulation (CPX-351) was FDA approved for upfront treatment of s-AML based on a pivotal phase 3 trial demonstrating improved overall survival in pts aged 60-75 years old (Lancet J et al; JCO 2018). Although CPX-351 treatment is indicated in all adults with s-AML, it is unclear whether CPX-351 is safe and effective in younger pts < 60 years. We sought to address this unanswered question by retrospective review of clinical experience since FDA approval at 4 large academic centers.
Methods: Medical records were retrospectively reviewed at Roswell Park Comprehensive Cancer Center, Moffitt Cancer Center, University of Alabama Comprehensive Cancer Center, and Levine Cancer Institute to identify pts aged 18-59 years old with untreated s-AML defined as antecedent MDS or CMML, prior cytotoxic therapy, or AML with WHO defined myelodysplasia related changes (AML-MRC) treated with CPX-351 as induction therapy. Demographics, disease-specific variables, as well as overall outcomes were collected in accordance with the institutional review board approved protocol. Responses were defined according to the 2003 International Working Group (IWG) criteria. Demographics, baseline clinical characteristics, treatment response, and adverse events were analyzed using descriptive statistics. Overall survival was estimated utilizing Kaplan-Meier (KM) analysis.
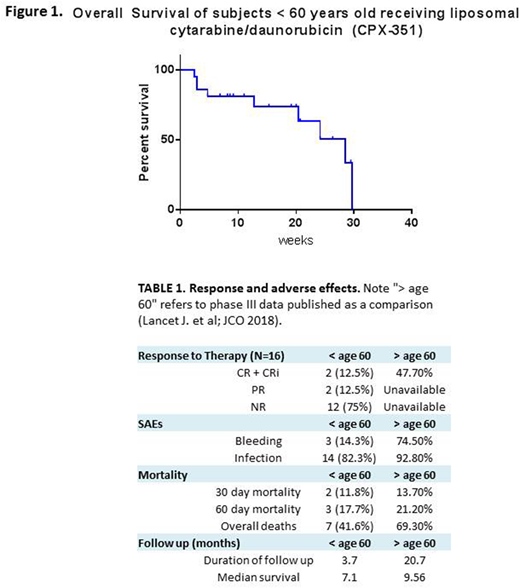
Results: Twenty-one pts with confirmed s-AML received CPX-351 therapy. Mean age was 54 years (range 42 - 59), 13 were male (62%). The majority (62%, N=13) had AML-MRC, 4 (19%) had treatment-related AML (t-AML) and 4 (19%) had MDS-MRC. Four of 5 pts had received prior hypomethylating therapy. Fourteen pts had a complex karyotype (67%), and 4 patients were found to have a normal karyotype (12%). The most common molecular event was TP53 mutation seen in 9 pts (43%), followed by FLT3-ITD identified in 3 pts (14%). At the time of analysis, response assessments were available for 16 pts. Overall response rate (CR/CRi/PR) was 25% with 1 CR (6.25%, 1/16), 1 CRi (6.25%, 1/16), and 2 PR (13%, 2/16). The remaining pts (12/16, 75%) were non-responders (Table 1). One pt has received an allogenic stem cell transplant. The most common adverse event was infection (81%, 17/21) with 3 clinically significant bleeding events. Thirty-day mortality was 14.3%, with 60-day mortality of 19.1%. Overall survival in all evaluable pts (N=21) was 7.1 months (range 0.5 - 7.4 months) (Figure 1), with mean follow up of 14.8 weeks.
Conclusions: This multi-institutional retrospective analysis suggests that CPX-351 results in lower response rates (CR/CRi 12.5%) and shorter overall survival (7.1 mos) than reported in the recently published phase 3 trial data in pts aged 60-75 years old (Table 1). Potential explanations for this discrepancy include short follow up, small sample size, the retrospective design of this study, and the significant proportions of pts with complex karyotype and TP53 mutations. Historically, patients < 65 years old with s-AML have had a reported overall survival of approximately 7 months. Further investigation of this regimen in younger pts with s-AML as compared with 7+3 and other approaches is warranted.
Thota:Incyte: Speakers Bureau. Baron:Pfizer Pharmaceuticals: Other: Previously served as a consult on the Advisory Boards (May 2017).. Griffiths:Alexion Inc.: Honoraria, Research Funding; Novartis, Inc.: Research Funding; Astex/Otsuka Pharmaceuticals: Honoraria, Research Funding; Pfizer, Inc.: Research Funding; Celgene, Inc: Honoraria, Research Funding. Sweet:Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria; Incyte: Research Funding; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Speakers Bureau; Agios: Consultancy; Celgene: Speakers Bureau. Wang:Jazz: Speakers Bureau; Jazz: Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Novartis: Speakers Bureau; Novartis: Speakers Bureau; Amgen: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal